Metabolomics Core Facility
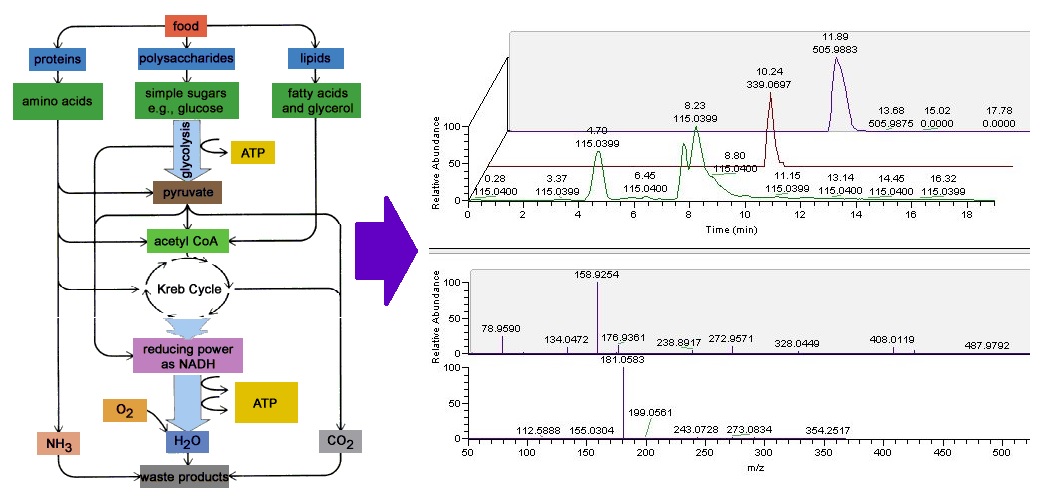
The mission of Metabolomics Core Facility (MCF) at Robert H. Lurie Comprehensive Cancer Center of Northwestern University is to provide: 1. LCMS based metabolomics service including identification, semi-quantification and quantification of metabolites from biological samples including but not limited to cells, bio-fluids and tissues; 2. integrative genomics service related to metabolism. MCF supports investigators engaged in basic, preclinical and clinical cancer research, including scientists examining basic mechanisms in of disease, as well as those seeking to identify novel targets for therapy or biomarkers that can be used for early detection, diagnosis, prognosis or response to therapy.
Contact Us
Metabolomics
- Olson 8-310
710 N. Fairbanks Court
Chicago, IL 60611 - 312-908-8312
- metabo@northwestern.edu
Integrative Genomics
- Simpson Querrey 5-312
303 E Superior St.
Chicago, IL 60611 - 312-503-0534
- Metabo.Genomics@northwestern.edu
Services and FAQs
Core Introduction & Metabolomics 101
Key Services
CHMP – Profiling (Semi-Quantitation) – Comprehensive Hydrophilic Metabolites Panel
Use LCMS to acquire signals from a broad spectrum of water-soluble metabolites. 300 plus targets include but not limit to amino acids, nucleotides and nucleobase/nucleosides, cofactors, Krebs cycle, TCA cycle, urea cycle, glycolysis, gluconeogenesis, pentose phosphate pathway, etc. See CHMP Target list for details. 30-40 minutes run with raw data available.
CLP – Profiling (Semi-Quantitation) – Comprehensive Lipids Panel
Use LCMS to acquire signals from a broad spectrum of lipids. 200 plus targets include CE, Cer, DG, LPC, PC,PE, PG, PI, PS, SM, TG, etc. See CLP Target List for details. 30-40 minutes run with raw data available.
PTM – Profiling (Semi-Quantitation) – Targeted Metabolites/Lipids/Small Molecules
Use LCMS to acquire signals for specific targets-of-your-interest (hydrophilic metabolites, lipids or other small molecules). Standard compounds are required for certain metabolites. 25-35 minutes run with raw data available.
FLUX – Profiling (Semi-Quantitation) – Fluxomics
Use LCMS to acquire signals of steady isotope (13C, 2H, or 15N) incorporated metabolites after incubation of substrates in order to study metabolic flux in specific pathways. Common substrates include but not limit to Glucose-13C6, Glutamine-13C5, Pyruvate-13C3, Creatine-13C4, Methionine-13C5, Nictinamide-13C3,15N. 25-35 minutes run with raw data.
QTM – Quantification (Absolute-Quantitation) – Targeted Metabolites/Lipids/Small Molecules
Use LCMS to quantify targets-of-your-interest (hydrophilic metabolites, lipids or small molecules) from sample extractions. Standard compounds of targets and internal standards (normally isotope labelled targets) are required.
UMHM – Untargeted Metabolomics (Discovery-Mode) – Hydrophilic Metabolites
Use LCMS to acquire signals from water soluble metabolites extractions. Raw data from untargeted metabolomics method (full MS1 scan with data dependent MS2) will be provided. Researchers will choose their own choice of partners (including core’s collaborator from outside institute) to perform untargeted metabolomics analysis.
- For profiling (Semi-Quantitation) services, targets will be annotated, and peak areas with artificial unit will be reported for comparison between samples.
- For quantification (Absolute-Quantitation) services, amount or concentration will be reported.
- For untargeted metabolomics (Discovery-Mode) services, only raw data with full MS1 scan and data dependent MS2 will be provided.
Sample Preparation
Process samples for LCMS injection.
Method Development
Develop or optimize new assays per customer request.
SeaHorse Analyzers
Shared instruments are provided for researchers to perform self-guided SeaHorse experiments.
Consulting
Sample Submission Process
- Please contact the correct core branch to discuss the project and submission.
- Once ready to submit samples, visit https://nucore.northwestern.edu/facilities/Metabo and follow the instructions to generate NUCore order.
- Print out the Sample Submission Form generated by NUCore ordering system and bring samples to core.
FAQs
Starting Materials - It is important to keep the amount of starting material (cell number, equivalent protein amount, bio-liquid volume and tissue weight, etc.) as same as possible across the batch. Starting material amount is required during submission to calculate injection amount.
Turnaround Time - Under normal circumstances, 10 business days are typical for medium-low complexity projects. Projects that are more complex take longer.
Sample/Data Storage - Leftover samples (if any) will be kept in -20°C freezer for 3 months, and the raw data will be kept for up to 12 months.
Approved Price List - Please contact core.
Equipment
- UHPLC-MS (Hybrid Quadrupole-Orbitrap): Thermo Ultimate-3000 + Q-exactive
- UHPLC-MS (Triple Quadrupole): Thermo Vanquish + TSQ
- SeaHorse Analyzer: XFe96 – 96-well plate
- SeaHorse Analyzer: XFp – 8-well plate
- Speed-Vac
Selected Publications
Myeloid Fatty Acid Metabolism Activates Neighboring Hematopoietic Stem Cells to Promote Heart Failure With Preserved Ejection Fraction.
Filipp M, Ge ZD, DeBerge M, Lantz C, Glinton K, Gao P, Smolgovsky S, Dai J, Zhao YY, Yvan-Charvet L, Alcaide P, Weinberg SE, Schiattarella GG, Hill JA, Feinstein MJ, Shah SJ, Thorp EB.
Metformin targets mitochondrial complex I to lower blood glucose levels.
Reczek CR, Chakrabarty RP, D'Alessandro KB, Sebo ZL, Grant RA, Gao P, Budinger GR, Chandel NS.
Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment.
Hunt EG, Hurst KE, Riesenberg BP, Kennedy AS, Gandy EJ, Andrews AM, Del Mar Alicea Pauneto C, Ball LE, Wallace ED, Gao P, Meier J, Serody JJ, Coleman MF, Thaxton JE.
Time-restricted feeding mitigates obesity through adipocyte thermogenesis.
Hepler C, Weidemann BJ, Waldeck NJ, Marcheva B, Cedernaes J, Thorne AK, Kobayashi Y, Nozawa R, Newman MV, Gao P, Shao M, Ramsey KM, Gupta RK, Bass J.
Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway.
Soflaee MH, Kesavan R, Sahu U, Tasdogan A, Villa E, Djabari Z, Cai F, Tran DH, Vu HS, Ali ES, Rion H, O'Hara BP, Kelekar S, Hallett JH, Martin M, Mathews TP, Gao P, Asara JM, Manning BD, Ben-Sahra I, Hoxhaj G.
IDH3α regulates one-carbon metabolism in glioblastoma.
May JL, Kouri FM, Hurley LA, Liu J, Tommasini-Ghelfi S, Ji Y, Gao P, Calvert AE, Lee A, Chandel NS, Davuluri RV, Horbinski CM, Locasale JW, Stegh AH.
Acknowledgement
Work performed in Metabolomics Core Facility should be acknowledged as following:
- Metabolomics experiments were performed by Metabolomics Core Facility at Robert H. Lurie Comprehensive Cancer Center (generously supported by NCI CCSG P30 CA060553) of Northwestern University.
Core Navigator
 For guidance on which cores may be most useful for your research and to coordinate use, please use the AI-powered Core Navigator chatbot, use the information request form or contact Sara Fernandez Dunne at s-fernandez@northwestern.edu or 847.491.5960.
For guidance on which cores may be most useful for your research and to coordinate use, please use the AI-powered Core Navigator chatbot, use the information request form or contact Sara Fernandez Dunne at s-fernandez@northwestern.edu or 847.491.5960.


